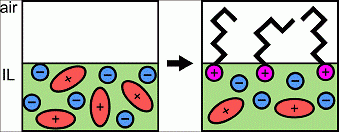
 Bermudez at the UMass Materials Research Science and Engineering Center found that room-temperature ionic liquids (ILs) exhibit a unique set of properties due to their charged character, presenting opportunities for applications not possible using conventional organic solvents or water. This work showed combining positively or negatively charged molecules with ILs resulted in previously unknown interfacial behavior due to the electrostatic interactions between the charged molecule and the liquid. Specifically, sodium alkyl sulfates (negatively charged surfactants) and alkyl trimethylammonium bromides (positively charged surfactants) were found to segregate to the air-IL interface, and x-ray photoelectron spectroscopy revealed that the surfactant counter ions readily dissociate into the bulk, rather than staying close-by their oppositely charged partner. In addition, for an IL with an initial negative surface charge, the charge could be switched to positive by the addition of alkyl trimethylammonium bromides, giving access to a new method of manipulating surface charge of materials, which could have striking consequence as applied to coatings, ultra-thin films, and anti-fouling surfaces.
Bermudez at the UMass Materials Research Science and Engineering Center found that room-temperature ionic liquids (ILs) exhibit a unique set of properties due to their charged character, presenting opportunities for applications not possible using conventional organic solvents or water. This work showed combining positively or negatively charged molecules with ILs resulted in previously unknown interfacial behavior due to the electrostatic interactions between the charged molecule and the liquid. Specifically, sodium alkyl sulfates (negatively charged surfactants) and alkyl trimethylammonium bromides (positively charged surfactants) were found to segregate to the air-IL interface, and x-ray photoelectron spectroscopy revealed that the surfactant counter ions readily dissociate into the bulk, rather than staying close-by their oppositely charged partner. In addition, for an IL with an initial negative surface charge, the charge could be switched to positive by the addition of alkyl trimethylammonium bromides, giving access to a new method of manipulating surface charge of materials, which could have striking consequence as applied to coatings, ultra-thin films, and anti-fouling surfaces.


