Polymers in Ionic Liquids
Coordinators: David Hoagland and Tom McCarthy.
Participants: Harry Bermudez, Shaw Ling Hsu, Alan Lesser, and Jim Watkins
The Center supported a Superseed project, Polymers in Ionic Liquids, which uncovered previously unknown features of the behavior of polymers in contact with ionic liquids (ILs).
ILs are salts that melt near or below room temperature, and serve as ambient temperature solvents composed entirely of ions and thereby possessing unique properties. ILs can dissolve, swell, or wet polymers, generating new materials and providing insight into unresolved questions about polymer materials that cannot be addressed using conventional aqueous and organic solvents. Distinguishing properties of ILs include negligible volatility, high conductivity, persistence as liquids over an extreme temperature range, and widely tunable solvency (i.e., highly variable dielectric constant, hydrogen bonding, and ion pairing). Many 'intractable' polymers such as cellulose and poly(vinyl alcohol) readily dissolve in ILs, enabling facile functionalization that is otherwise problematic in aqueous media or organic solvents. ILs display new properties - as a strongly associated two-component liquid, ILs separate into cationic and anionic domains of sizes similar to polymer coils. Moreover, one IL component may enrich at a liquid interface, altering the structure and properties of interfacially active polymers. IL properties depend on the identities of the cation and anion, and numerous new cationic/anionic IL pairings have been reported, yielding a wide array of solvents with immense variation in properties.
Exploiting negligible IL volatility
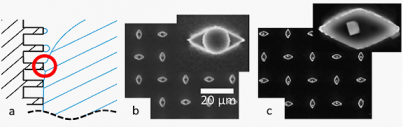 SuperSeed research seeks to image 'wet' polymer systems, enabled by the negligible volatility of ILs that makes them compatible with the high vacuum of the electron microscope. In situ visualization of static and dynamic structure thereby becomes feasible, circumventing limitations and artifacts inherent to techniques such as cryo-TEM. Thus, electron microscopy is envisaged as an insightful tool to image polymer and solvent (fluid) statics and dynamics at the nano- and micro-scale, a topic with little precedent. Figure 1 displays wetting behavior near the receding contact line of a liquid droplet placed on an ultralyophobic surface presenting an array of hydrophobized micron-sized "posts". Because of the hydrophobicity, the IL does not penetrate between the posts but rather is localized atop the posts. ILs placed on such an array display high advancing contact angels, and nearly identical advancing and receding angles. Consequently, under a minimal lateral force, an IL droplet moves rapidly across the surface by sliding or rolling. The sketch of Fig 1a depicts wetting dynamics near the tail edge of such a droplet, the liquid receding by sequential formation and breakage of capillary bridges. With conventional solvents, validating this depiction would be nearly impossible, since evaporation is faster than optical or SEM imaging. However, with ILs, submicron droplets are visualized atop each post after the contact line has passed, as shown in Fig 1b, validating the proposed mechanism. Exploiting this discovery, the IL was replaced with an aqueous salt solution that wets the surface analogously, in which solvent evaporation leaves a small salt crystal on each post, as shown in Fig 1c.
SuperSeed research seeks to image 'wet' polymer systems, enabled by the negligible volatility of ILs that makes them compatible with the high vacuum of the electron microscope. In situ visualization of static and dynamic structure thereby becomes feasible, circumventing limitations and artifacts inherent to techniques such as cryo-TEM. Thus, electron microscopy is envisaged as an insightful tool to image polymer and solvent (fluid) statics and dynamics at the nano- and micro-scale, a topic with little precedent. Figure 1 displays wetting behavior near the receding contact line of a liquid droplet placed on an ultralyophobic surface presenting an array of hydrophobized micron-sized "posts". Because of the hydrophobicity, the IL does not penetrate between the posts but rather is localized atop the posts. ILs placed on such an array display high advancing contact angels, and nearly identical advancing and receding angles. Consequently, under a minimal lateral force, an IL droplet moves rapidly across the surface by sliding or rolling. The sketch of Fig 1a depicts wetting dynamics near the tail edge of such a droplet, the liquid receding by sequential formation and breakage of capillary bridges. With conventional solvents, validating this depiction would be nearly impossible, since evaporation is faster than optical or SEM imaging. However, with ILs, submicron droplets are visualized atop each post after the contact line has passed, as shown in Fig 1b, validating the proposed mechanism. Exploiting this discovery, the IL was replaced with an aqueous salt solution that wets the surface analogously, in which solvent evaporation leaves a small salt crystal on each post, as shown in Fig 1c.
ILs and proteins
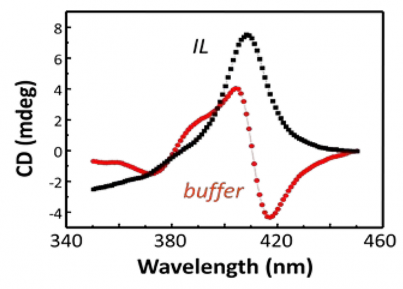 Hoagland, Emrick, and Bermudez have demonstrated the value of using ILs in conjunction with proteins, investigating for example the conformation and activity of proteins in IL environments. Dissolving proteins in ILs opens numerous new opportunities, including improved polymer-protein conjugation strategies and stable environments for long-term room-temperature protein storage. Fig 2 shows the Soret region of the circular dichroism (CD) spectra of the protein cytochrome-c in aqueous buffer (red curve) and in the hydrophilic IL [EMIM][EtSO4] (black curve). In this IL, the protein's heme unit lacks the expected axial ligand, causing local denaturation. Interestingly, the protein retains its secondary structure and its native activity, suggesting new and unexpected structure/function relationships arising from IL-protein combinations. ILs also prove useful for providing non-aqueous media for polymer-protein conjugation chemistries typically performed in aqueous buffer. Classic reactive functional groups, such as N-hydroxysuccinimidyl (NHS) esters, are hydrolytically unstable, often resulting in poor conjugation efficiencies. However, polymer modification of proteins can be accomplished using polymer zwitterions in ILs. This is particularly useful, since protein conjugation to extremely hydrophilic polymers, such as polymer zwitterions, limits solvent choices to water and methanol, and a problematic loss of reactive chain-ends such as NHS esters, rendering them unavailable for protein conjugation. Employing ILs allows for retention of the end-groups and thus high conjugation efficiency. Lysozyme was chosen as a model enzyme to illustrate the concept. A fluorescence-based assay demonstrated that polyMPC-lysozyme conjugates retain the activity inherent to the native enzyme. Moreover, native lysozyme was also shown to retain high activity even after exposure to IL.
Hoagland, Emrick, and Bermudez have demonstrated the value of using ILs in conjunction with proteins, investigating for example the conformation and activity of proteins in IL environments. Dissolving proteins in ILs opens numerous new opportunities, including improved polymer-protein conjugation strategies and stable environments for long-term room-temperature protein storage. Fig 2 shows the Soret region of the circular dichroism (CD) spectra of the protein cytochrome-c in aqueous buffer (red curve) and in the hydrophilic IL [EMIM][EtSO4] (black curve). In this IL, the protein's heme unit lacks the expected axial ligand, causing local denaturation. Interestingly, the protein retains its secondary structure and its native activity, suggesting new and unexpected structure/function relationships arising from IL-protein combinations. ILs also prove useful for providing non-aqueous media for polymer-protein conjugation chemistries typically performed in aqueous buffer. Classic reactive functional groups, such as N-hydroxysuccinimidyl (NHS) esters, are hydrolytically unstable, often resulting in poor conjugation efficiencies. However, polymer modification of proteins can be accomplished using polymer zwitterions in ILs. This is particularly useful, since protein conjugation to extremely hydrophilic polymers, such as polymer zwitterions, limits solvent choices to water and methanol, and a problematic loss of reactive chain-ends such as NHS esters, rendering them unavailable for protein conjugation. Employing ILs allows for retention of the end-groups and thus high conjugation efficiency. Lysozyme was chosen as a model enzyme to illustrate the concept. A fluorescence-based assay demonstrated that polyMPC-lysozyme conjugates retain the activity inherent to the native enzyme. Moreover, native lysozyme was also shown to retain high activity even after exposure to IL.


